Isolated non-compaction cardiomyopathy (INCCM) and its typical echocardiographic appearance were first described in 1984 by Engberding and Bender. INCCM is a heart-muscle disorder that is still little known among physicians. In a study of children with primary cardiomyopathy of all types, NCCM was present in 9.2%. It was thus the third most common type of primary cardiomyopathy, after dilated cardiomyopathy (DCM) and hypertrophic cardiomyopathy.
Noncompaction of the ventricular myocardium is a cardiomyopathy thought to be caused by arrest of normal embryogenesis of the endocardium and myocardium. This abnormality is often associated with other congenital cardiac defects, but it is also seen in the absence of other cardiac anomalies. Clinical manifestations are highly variable, ranging from no symptoms to disabling congestive heart failure, arrhythmias, and systemic thromboembolism. Echocardiography has been the diagnostic procedure of choice, but the correct diagnosis is often missed or delayed because of lack of knowledge about this uncommon disease and its similarity to other diseases of the myocardium and endocardium.
Embryology and Development:
During early embryonic development, the myocardium is a loose network of interwoven fibers separated by deep recesses that link the myocardium with the left ventricular cavity. Gradual “compaction” of this spongy meshwork of fibers and intertrabecular recesses, or “sinusoids,” occurs between weeks 5 and 8 of embryonic life, proceeding from the epicardial to endocardium and from the base of the heart to the apex. The coronary circulation develops concurrently during this process, and the intertrabecular recesses are reduced to capillaries.
The normal process of trabeculation appears to involve secretion of neuregulin growth factors from the endocardium and may also involve angiogenesis factors, such as vascular endothelial growth factor and angiopoietin.
Noncompaction of the ventricular myocardium (NVM) is an uncommon finding. It is thought to be caused by arrest of the normal process of endomyocardial morphogenesis.
NCCM was first described in association with other congenital anomalies, such as obstruction of the right or left ventricular outflow tracts, complex cyanotic congenital heart disease, and coronary artery anomalies. The abnormal compaction process in these cases is not fully understood, but pressure overload or myocardial ischemia preventing regression of the embryonic myocardial sinusoids has been suggested. This process results in the persistence of deep intertrabecular recesses in communication with both the ventricular cavity and the coronary circulation.
Isolated non-compaction cardiomyopathy (INCCM) and its typical echocardiographic appearance were first described in 1984 by Engberding and Bender is characterized by persistent embryonic myocardial morphology found in the absence of other cardiac anomalies to explain the abnormal development. In such cases, the resultant deep recesses communicate only with the ventricular cavity, not the coronary circulation.
The left ventricle is uniformly affected, but biventricular noncompaction has been reported, with right ventricular noncompaction described in less than one-half of patients. Because of difficulty in distinguishing normal variants in the highly trabeculated right ventricle from the pathological noncompacted ventricle, several authors dispute the existence of right ventricular noncompaction.
Both familial and sporadic forms of noncompaction have been described.
In the original report of INCCM, which predominantly involved children, familial recurrence was seen in half of patients. Familial recurrence was seen in 18% in the largest reported adult population with INCCM, although the authors reported that incomplete screening of siblings may account for the lower percentage compared with the earlier report.
Epidemiology:
The prevalence of this disease in adults remains unclear. In observational studies, NCCM has been found in 0.014% to 0.26% of all adults referred to an echocardiography laboratory. The incidence of NCCM in the general population has been estimated at 0.05% to 0.25% per year. The diagnosis is presumably often missed, because the disease is still not as well-known as it should be among physicians at large.
Clinical Features and Pathophysiology:
Three major clinical manifestations of noncompaction have been described, including: Heart failure, arrhythmias, and embolic events.
Findings vary among patients, ranging from asymptomatic left ventricular dysfunction to severe, disabling congestive heart failure.
Over two thirds of the patients in the largest series with NCCM had symptomatic heart failure.
Coronary angiography reveals no abnormalities in patients with NCCM, but positron emission tomography (PET) shows a diminished reserve of coronary blood flow in the compact and non-compact myocardial segments of the left ventricle, presumably because of impaired microcirculation. Similar findings are obtained with single photon emission computerized tomography (SPECT).
Depressed ventricular systolic function has been noted in 63% of patients. Both systolic and diastolic ventricular dysfunction has been described.
Restrictive hemodynamics by cardiac catheterization, as well as an initial presentation of NCCM as a restrictive cardiomyopathy, have been described in children with NCCM.
Left ventricular dysfunction developed in the vast majority, regardless of the presence or absence of symptoms at initial diagnosis.
Furthermore, marked trabeculation can impair the diastolic function of the left ventricle as well, with abnormal relaxation and restricted filling.
The origin of systolic dysfunction in noncompaction is unclear, but it has been suggested that subendocardial hypoperfusion and microcirculatory dysfunction playing roles in ventricular dysfunction and arrhythmogenesis.
These systolic and diastolic disturbances of left ventricular function, if severe enough, can lead to the clinical manifestations of heart failure that are seen in many patients with NCCM.
Arrhythmias are common in patients with ventricular noncompaction. Atrial fibrillation has been reported in over 25% of adults with NCCM. Ventricular tachyarrhythmia has been reported in as many as 47%. Sudden cardiac death accounted for half of the deaths in the larger series of patients with NCCM. Paroxysmal supraventricular tachycardia and complete heart block have also been reported in patients with NCCM.
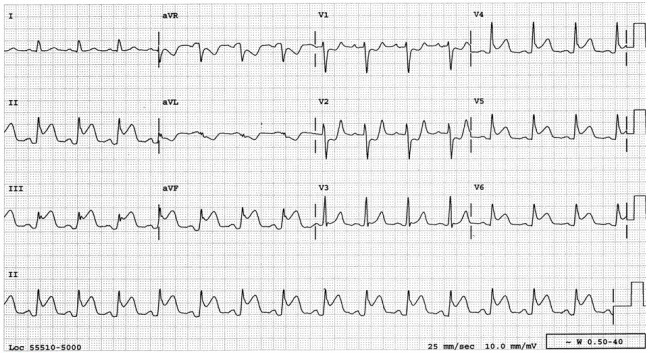
Abnormalities of the resting ECG are found in the majority of patients with NCCM but findings are nonspecific and include left ventricular hypertrophy, repolarization changes, inverted T waves, ST segment changes, axis shifts, intraventricular conduction abnormalities, and AV block. Left bundle branch block has been described in 44% of adult patients with NCCM, but the reported incidence in children was much lower.
Electrocardiographic findings of the Wolff-Parkinson-White syndrome have been described in up to 15% of pediatric patients.
The occurrence of thromboembolic events, including cerebrovascular accidents, transient ischemic attacks, pulmonary embolism, and mesenteric infarction, ranged from 21% to 38%.
Embolic complications may be related to development of thrombi in the extensively trabeculated ventricle, depressed systolic function, or the development of atrial fibrillation. Of interest, no systemic embolic events were reported in the largest pediatric series with NCCM.
An association between NCCM and facial dysmorphisms, including a prominent forehead, low-set ears, strabismus, high-arching palate, and micrognathia, has been described.
Diagnosis:
Echocardiography is the diagnostic test of choice for NCCM. Multiple prominent ventricular trabeculations with deep intertrabecular recesses are seen. Color Doppler imaging demonstrates blood flow through these deep recesses in continuity with the ventricular cavity.
The left ventricular apical and inferior wall segments were involved in all patients in an adult population with NCCM studied by echocardiography. The right ventricular apex was involved in 41%.
Depressed left ventricular systolic function was the rule, with a mean calculated ejection fraction of 33% in 28 patients examined. Impairment of diastolic function as assessed by mitral inflow and pulmonary venous flow Doppler was observed in all, including a restrictive filling pattern in 36%.
Prominent trabeculations (although virtually always < 3 in number in normal variants), apical hypertrophic cardiomyopathy, dilated cardiomyopathy, arrhythmogenic right ventricular dysplasia, endocardial fibroelastosis, cardiac metastases, and left ventricular thrombus are important differential diagnostic considerations. Transesophageal echocardiography may be used when transthoracic studies cannot reliably exclude other processes. One report described the use of contrast echocardiography with sonicated albumin in a patient with NCCM. Contrast echocardiography may be helpful when standard echocardiographic image quality is limited or the diagnosis is questionable.
Cardiac magnetic resonance imaging (MRI) is a further method that can be used to diagnose NCCM accurately. Cardiac magnetic resonance imaging has become the method of choice to confirm or rule out left ventricular noncompaction, because echocardiography cannot allow proper visualisation of the apex in some cases.
MRI provides good correlation with echo for localization and extent of noncompaction and is useful in cases with poor echocardiographic image quality.
In addition, the demonstration of differences in MRI signal intensity in noncompacted myocardium may help identify substrate for potentially lethal arrhythmias.
Management:
Treatment for noncompaction of the ventricular myocardium focuses on the 3 major clinical manifestations: Heart failure, arrhythmias, and systemic embolic events. Standard medical therapy for systolic and diastolic ventricular dysfunction is warranted. Cardiac transplantation has been used for those with refractory congestive heart failure.
The beneficial effects of the beta blocker carvedilol on left ventricular function, mass, and neurohormonal dysfunction in an infant with NCCM have been described. Because of the frequency of ventricular tachycardia and significant risk of sudden cardiac death and systemic embolism, assessment for atrial and ventricular arrhythmias by ambulatory ECG monitoring should be performed annually. As more information is gathered about NCCM and risk of sudden cardiac death, implantable defibrillator technology may have an expanded role. Biventricular pacemakers may have a role in the treatment of NCCM patients with heart failure, reduced left ventricular function, and prolonged intraventricular conduction.
Prevention of embolic complications is also an important management issue, and several authors have recommended long-term prophylactic anticoagulation for all patients with ventricular noncompaction whether or not thrombus has been found. Because of the familial association described with noncompaction, screening echocardiography of first degree relatives is recommended. Given the high percentage of associated neuromuscular disorders reported in patients with NCCM, neurological and musculoskeletal evaluations are also recommended.
Prognosis:
Although the prognosis for patients with NCCM varies, nearly 60% of patients described in one large series had either died or undergone cardiac transplantation within 6 years of diagnosis.
Two of 8 in the initially asymptomatic group of this series died during the follow-up period, both having documented sustained ventricular tachycardia and one with sudden cardiac death. Similarly, in a series of 34 adults with NCCM, 47% either died or underwent cardiac transplantation during the follow-up period of 44 months.
The occurrences of systemic emboli, ventricular arrhythmias, and death were considerably lower in the largest pediatric series with NCCM when compared with adults and the initial case series by Chin et al, although nearly 90% of patients followed for up to 10 years developed left ventricular dysfunction.
Oechslin et al reported that certain clinical characteristics were observed significantly more frequently in nonsurvivors compared with survivors with NCCM, including:
1) – Higher left ventricular end diastolic diameter on presentation.
2) – New York Heart Association class III-IV.
3) – Permanent or persistent atrial fibrillation.
4) – Bundle branch block.
Patients with these high risk features are candidates for early, aggressive interventions, including consideration of cardioverter-defibrillator implantation and evaluation for transplantation.
Conclusion:
Non-compacted cardiomyopathy is a congenital malformation that can occur as an isolated entity or associated with other pathologies of the heart and can often involve both ventricles. A ratio of noncompacted: compacted wall greater than 3 and involvement of three or more segments are signs of poor prognosis associated with greater clinical deterioration (functional class III/IV) and ventricular arrhythmias.
A high index of suspicion for non-compaction is necessary as the condition may lead to serious heart failure, thromboembolic events, ventricular tachyarrhythmia or death. Early recognition of non-compaction may give better follow-up and management of patients with this condition.
The clinical picture does not provide sufficiently specific evidence to establish the diagnosis. Echocardiography is the diagnostic cornerstone. Treatment should be directed towards prevention and management of heart failure, ventricular arrhythmias and prevention of thromboembolic events. The long-term prognosis of non-compaction cardiomyopathy is poor.
Unresolved issues:
- Definitive echocardiographic and CMR criteria for diagnosis.
- Prevalence of RV involvement and diagnostic criteria.
- Whether prognosis can be improved by early diagnosis-treatment.
- Comprehension of the poor genotype-phenotype correlation.
Suggested Readings:
1) – Weiford B.C., et al; Noncompaction of the Ventricular Myocardium. Circulation 2004, 109:2965-2971.
2) – Engberding R, et al. Isolated Non-compaction Isolated Cardiomyopathy. Dtsch Arztebl Int. 2010 March; 107(12): 206–213.
3) – Patil VC, Patil HV. Isolated Non-compaction Cardiomyopathy presented with Ventricular Tachycardia. Hear Views, 2011 Apr-Jun; 12(2):74-78